FDA set to authorize Pfizer vaccine for ages 12 and above

Photo contributed by Sophia Papp ’24, Graphic by Claire Redmer ’21
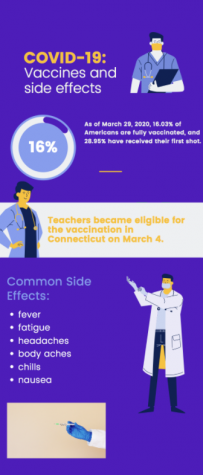
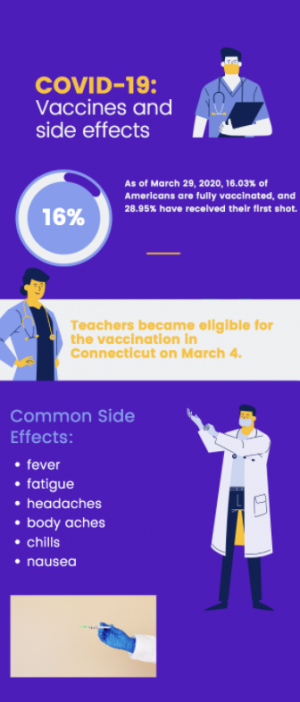
The FDA is predicted to approve the distribution of the Pfizer-BioNTech COVID-19 vaccine for adolescents ages 12 to 15 early next week. Connecticut plans to get vaccines to this age group through the use of school clinics.
The Food and Drug Administration (FDA) is set to authorize distribution of the Pfizer-BioNTech COVID-19 vaccine to 12 to 15-year-olds during the week of May 10.
The vaccine was previously approved for those 16 and older; however, recent clinical trials showed the efficacy in 12 to 15-year-olds was similar to that in adults.
“Pfizer reported several weeks ago that none of the adolescents in the clinical trial who received the vaccine developed symptomatic infections, a sign of significant protection,” the New York Times wrote in an article regarding the pending FDA approval of the Pfizer vaccine for 12 to 15 year-olds. “The company said that volunteers produced strong antibody responses and experienced about the same side effects seen in people ages 16 to 25.”
Once approved, Connecticut Governor Ned Lamont plans to expedite the vaccine rollout to younger age groups through the use of school clinics, according to an article on Patch.com. The state hopes to have such clinics in time for the school year, stating that these would help build confidence in a safe return to in-person learning.
Younger Staples students expressed excitement regarding the expected vaccine approval.
“I am excited and relieved that the Pfizer vaccine is soon going to be available for 12 to 15-year-olds,” Sophia Papp ’24 said. “Personally, I am planning on getting the vaccine. I think it is important to not only protect myself, but protect the people around me.”
The pending age group expansion was not without controversy, as some felt that available vaccines should be dedicated to people deemed more at-risk than adolescents. The New York Times states that some, including Dr. Rupali J. Limaye, a researcher at Johns Hopkins University studying vaccine use and hesitancy, feel that the U.S. should donate excess doses to countries currently experiencing outbreaks, such as India. Even if they hold on to the shots, Limaye said that adults should be the focus as health officials continue to develop personalized local outreach and vaccine distribution.
“We still need to go over hesitant adults while simultaneously, maybe starting at 14- or 15-year-olds,” Limaye said to the Times. “But the priority should still be adults.”